L'altro giorno, a pranzo, chiaccheravo con i colleghi.
Sembravano tutti patologi legali espertissimi, ma ho capito il trucco. Tra i vari teleplay CSI, RIS, gli omicidi risolti o meno da prima pagina puntualmente ripresi da Lucarelli, dalla Pivetti, da Vespa etc., non è difficile improvvisarsi Petrocelli in erba.
Ma a me interessa sempre di più il come funziona, fin da piccolo. Ho trovato un bell'articolo sul famigerato Luminol. Eccolo.
In this article, we'll find out how this strange compound, commonly known as luminol, reveals hidden crime scenes. As we'll see, this chemical is just as cool as it sounds, but it does have drawbacks and limitations not usually addressed on TV.
Much of crime scene investigation, also called criminalistics, is based on the notion that nothing vanishes without a trace. This is particularly true of violent crime victims. A murderer can dispose of the victim's body and mop up the pools of blood, but without some heavy-duty cleaning chemicals, some evidence will remain. Tiny particles of blood will cling to most surfaces for years and years, without anyone ever knowing they're there.
The basic idea of luminol is to reveal these traces with a light-producing chemical reaction between several chemicals and hemoglobin, an oxygen-carrying protein in the blood. The molecules break down and the atoms rearrange to form different molecules. In this particular reaction, the reactants (the original molecules) have more energy than the products (the resulting molecules). The molecules get rid of the extra energy in the form of visible light photons. This process, generally known as chemiluminescence, is the same phenomenon that makes fireflies and light sticks glow.
Investigators will spray a suspicious area, turn out all the lights and block the windows, and look for a bluish-green light. If there are any blood traces in the area, they will glow.
The "central" chemical in this reaction is luminol (C8H7O3N3), a powdery compound made up of nitrogen, hydrogen, oxygen and carbon. Criminalists mix the luminol powder with a liquid containing hydrogen peroxide (H2O2), a hydroxide (OH-) and other chemicals, and pour the liquid into a spray bottle. The hydrogen peroxide and the luminol are actually the principal players in the chemical reaction, but in order to produce a strong glow, they need a catalyst to accelerate the process. The mixture is actually detecting the presence of such a catalyst, in this case the iron in hemoglobin.
To perform a luminol test, the criminalists simply spray the mixture wherever they think blood might be. If hemoglobin and the luminol mixture come in contact, the iron in the hemoglobin accelerates a reaction between the hydrogen peroxide and the luminol. In this oxidation reaction, the luminol loses nitrogen and hydrogen atoms and gains oxygen atoms, resulting in a compound called 3-aminophthalate. The reaction leaves the 3-aminophthalate in an energized state -- the electrons in the oxygen atoms are boosted to higher orbitals. The electrons quickly fall back to a lower energy level, emitting the extra energy as a light photon. With iron accelerating the process, the light is bright enough to see in a dark room.
Investigators may use other chemiluminescent chemicals, such as fluorescein, instead of luminol. These chemicals work the same basic way, but the procedure is a little bit different.
If luminol reveals apparent blood traces, investigators will photograph or videotape the crime scene to record the pattern. Typically, luminol only shows investigators that there might be blood in an area, since other substances, including household bleach, can also cause the luminol to glow. Experienced investigators can make a reliable identification based on how quickly the reaction occurs, but they still need to run other tests to verify that it is really human blood.
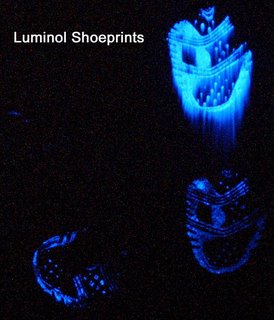
Luminol in itself won't usually solve a murder case. It's only one step in the investigative process. But it can reveal essential information that gets a stalled investigation going again. For example, hidden blood spatter patterns can help investigators locate the point of attack and even what sort of weapon was used (a bullet makes blood splatter very differently than a knife does). Luminol may also reveal faint bloody shoe prints, which gives investigators valuable information about the assailant and what he or she did after the attack.
In some cases, luminol leads investigators to more evidence. For example, if luminol detects trace amounts of blood on a carpet, investigators may pull up the carpet and discover a lot of visible blood on the floorboards below.
One problem with luminol is that the chemical reaction can destroy other evidence in the crime scene. For this reason, investigators only use luminol after exploring a lot of other options. It is definitely a valuable tool for police work, but it's not quite as prevalent in crime investigation as presented on some TV shows. The police don't walk into a crime scene and start spraying luminol on every visible surface.
Luminol Chemistry
5-amino-2,3-dihydro-1,4-phthalazine-dione, or luminol has become a commonly used, and functional compound. Luminol is a relatively simple chemical containing only carbon, nitrogen, oxygen and hydrogen. It was discovered in the late nineteenth century and later improved upon. Luminol is used often in biochemistry and serves many purposes from Halloween fun to crime scene blood detection.
Luminol, (C8H7N3O2) has quite a few other names; these include: 5-amino-2,3-dihydro-1,4-phthalazine-dione, o-aminophthalyl hydrazide, 3-aminophthathic hydrazide, and o-aminophthaloyl hydrazide1. Its molecular weight is 177.16, and has a melting point of 319ºC-320ºC. Its solubility is less than 0.1 grams per 100 milliliters at 19ºC, and looks like a yellow grainy substance. If reacted the luminol emits a green-blue light with varying intensity.
Luminol is a chemiluminescent compound, which means that a release of light is a result of a chemical reaction2. Fireflies are nature’s chemiluminescent creatures, they create a similar reaction to produce their own light source, (which can be seen at night in more rural areas.) This light is energy being released.
When luminol is placed in a basic solution such as perborate, permanganate, hyperchlorite, iodine, or hydrogen peroxide, and a catalyst such as iron, manganese, copper, nickel, or cobalt, the luminol is oxidized. (A catalyst is the most important ingredient to the reaction, because the stronger the catalyst, the longer and brighter the light will glow.) Most metals aid in the reaction, but there are a few that actually repress the response. Hydrogen peroxide works best as the base, it “burns” the luminol. Cobalt has proven to be the best metal catalyst. The luminol creates light via oxidation, because the two nitrogen atoms are easily replaced by two oxygen atoms. As this reaction occurs, nitrogen gas is discharged, leaving the luminol in an excited state, with additional energy which is then released as light. Amino acids, tegatose, fructose, glycerols, thiols, and serum albumin can also react with luminol to produce an intense light. No excitation source is needed to produce a glow, but a photomultiplier tube may be used to measure and detect the amounts of light.
In 1895, two scientists named Wiedemann and Schmid, dissolved cathode-ray irriaded alkali halides such as NaCl, NaBr, KCl, and KBr, in water. They noticed a very weak bluish light. They also detected light production when irriaded calcium carbonate was “attacked” by aqueous hydrochloracetic acid, or phosphoric acid.
Later in 1928, a chemist by the name of Albrecht discovered a specific chemical, that when placed in an aqueous alkaline solution emitted a blue-green light with a fair amount of intensity. Along with the light, virtually no heat was produced. This solution contained hydrogen peroxide along with a catalyst. The catalyst was an alkaline medium with a pH between ten and eleven. This specific chemical was to later on be called luminol.
Albrecht also determined the maximum intensity of light in this new chemical to be 424nm; he also concluded that fresh luminol was very unstable in its light yield, and the light yield itself came from the dissolved oxygen, and favored a trace metal. These discoveries led to a compound that created a useful cold light source at relative ease.
Halloween has always been a night of excitement for children, a night when they can pretend to be anything, and a night full of sugar. Many children carry green glow-sticks on Halloween to illuminate themselves for safety purposes. These light-sticks contain dilute H2O2 in a phthalic ester solvent in a capsule. This is the surrounded by a phenyl oxalate ester and 9,10-bis(phenylethynl) anthracene. When snapped, these two chemicals combine to produce luminol. The glow-sticks are green, because of other chemicals. At other events (such as fairs) multi-colored necklaces and bracelets are seen, and use this same type of compound to create their glow.
Often, luminol is used in biology and biochemistry for a multitude of testing. Chromatography, (which is a method for sorting chemical substances) immunoassay, (measures minute concentrations of biological matter in blood) DNA probes, and DNA fingerprinting, all use luminol as a testing reagent, and it is used as a substrate in western blot detection.
The most important and well-known use of luminol is in the field of forensic science. In 1937, a German forensic scientist, discovered the use of luminol in blood detection8. Blood, which is slightly alkaline, contains cells, water, enzymes, proteins, and hemoglobin9. Hemoglobin (which contains iron) carries oxygen to parts of the body. This hemoglobin reacts with the luminol as the catalyst10. Luminol can detect very small amounts of blood many years old.
Once an area is suspected to have blood (even if the area has been cleaned) luminol can be applied. The lights are turned out, and after a brief few seconds (approximately five seconds,) a glow may appear. Just because an area glows, does not necessarily mean blood is the culprit. Bleach, dyes, and other organic material can react with the luminol. For the most part, luminol is a very useful tool in murder and rape investigations9. Luminol has helped put many murderers and rapist in prison, when evidence seemed to be hidden.
Just because it can trace minuscule amounts of blood at an efficient manner, does not mean that it does not have any drawbacks in crime scene investigation. Because it can detect other chemicals and compounds, further testing is almost always required to determine if the reactant is blood. If so, then the process begins to establish the type of blood, and if it is the victim’s blood. If luminol is used, it can break down and cause a loss to several genetic markers used in genetic testing as well as destroy other important properties of the blood. While it can detect even small amounts of blood, the disadvantage is often that the small amount identified is diluted further by the luminol solution. For these reasons, luminol is encouraged to be used as a last resort in crime scenes to protect what little physical evidence is there already.
The chemiluminescent properties of luminol are remarkable in the fact that instead of heat, reactions involving luminol produce a cool light that has become a useful tool for both the scientific community and average individuals. It has aided research in blood. Luminol has helped to make the world a safer place by aiding the forensic community in its investigations. It has helped to further genetic testing via DNA probes, and medical testing, and has created an relatively safe natural light that can be seen anywhere on Halloween night.
Howstuffwork.com ©

0 Comments:
Post a Comment
<< Home